|
[Headlines] (20210614-의건운) US Drug Agency Approves Disputed Alzheimer’s Treatment
최고관리자 | 21-06-15 07:30
(1) "Write 2 Speak 전체 목록 & 모든 콘텐츠"를 보시려면 "Write 2 Speak = 유튜브 채널"에
가입(subscribe - 클릭)후 "본인의 유튜브 ID & Write2Speak 등록 ID"를 "write2speak@daum.net"로
이메일로 보내 주시면 확인 후 본 사이트 정회원으로 등급됩니다. 많은 신청 기다리겠습니다.
(2) 참고로, "Write 2 Speak"에 올라오는 유튜브 contents는 정기적으로 삭제가 되며, "전체 내용"을 보시려면
위에 절차를 따라야만 "Write 2 Speak -> 자료실(클릭)"영역에서 모든 내용을 다시 볼 수 있습니다.
****************************************************************************************
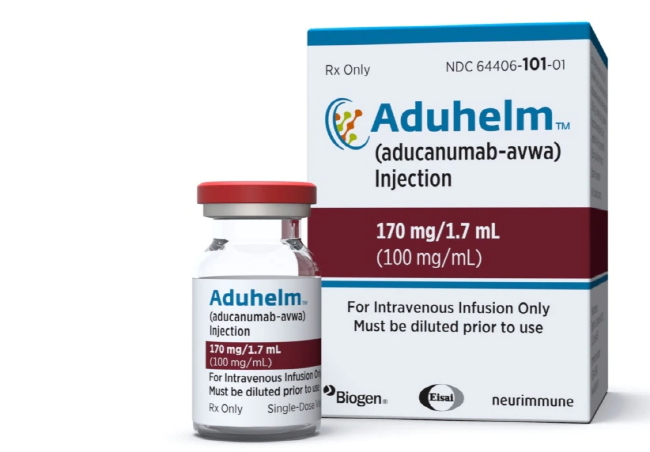
* US Drug Agency Approves Disputed Alzheimer’s Treatment
U.S. government health officials have approved the first new drug for Alzheimer’s disease in nearly 20 years. However, independent experts say the treatment has not been shown to help slow the brain disease, reported the Associated Press. Last week the Food and Drug Administration (FDA) approved the drug, called aducanumab. It was developed by the biotechnology company Biogen in Cambridge, Massachusetts. The drug will be sold under the name of Aduhelm. Biogen’s stock value increased by 38 percent on the day of the FDA approval.
   |